IPCUREB OWERVIEW
IP-cure-B is a five-year project started in January 2020, coordinated by INSERM in France.
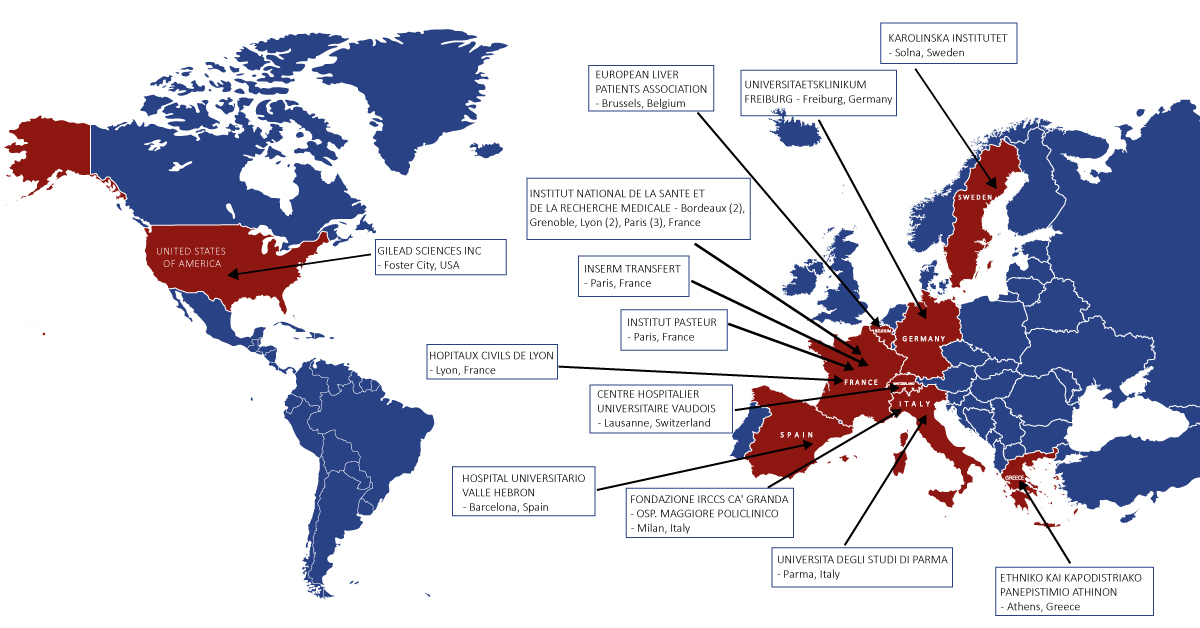
Our collaborative network brings together 13 complementary partners, including 10 research institutions, 1 pharmaceutical company, 1 patient association and 1 private company from 9 different countries (including 8 European member states). The project is funded by the European Commission under the H2020 Programme.
Members of the consortium
- Ethniko kai Kapodistriako Panepitimio Athinon
- Hospital Universitario Valle Hebron
- Gilead Sciences Inc.
- European Liver Patients Association
- Inserm Transfert
- Hôpitaux Civils de Lyon
- INSERM
- Centre hospitalier universitaire Vaudois
- Karolinska Institutet
- Institut Pasteur
- Università degli Studi di Parma
- Fondazione IRCCS ca’Granda
- Universitätsklinikum Freibur
Outcomes of the project
Outcomes for medical experts
The project aims at proposing a treatment paradigm shift with the development of novel combination therapies for CHB with improved cure rates and a better appraisal of novel biomarkers to improve patient selection for treatment and monitoring treatment response.
Outcomes for patient
The project aims at designing and developing novel treatment strategies towards the cure of infection with shorter duration of treatment. It is expected to result in improved quality of life and decreased social stigma.
Outcomes for community
The development of such curative therapies with finite duration of administration will pave the way towards universal treatment of all individuals that suffer from chronic HBV infection. It is thus expected to result in long-term reduction in cost and global burden of CHB (short-term therapy, increased treatment uptake in the infected population, decreased rate of liver complications and mortality).
IP-cure-B in numbers
- 13 partners : 10 institutes, 1 pharmaceutical companies, 1 patient association, 1 private company
- 9 different countries (8 EU member states)
- 9 983 031,56 M€ EC contribution
- 5 year-duration – start January 1 st , 2020