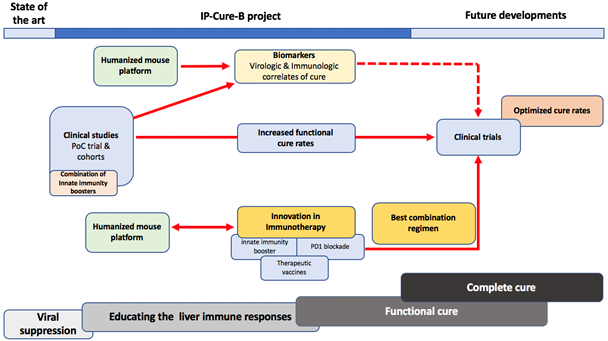
The IP-cure-B consortium, through the unique expertise of its members, proposes a completely novel research program investigating in pre-clinical models and in a proof of concept (PoC) study several complementary approaches aiming at:
1. Stimulating monocytes by TLR8 agonization to induce IL12 and IFN-gamma production that subsequently stimulate HBV-specific CD8 T cells;
2. Exploiting the immunomodulatory effects of NUC discontinuation to stimulate innate signaling and adaptive immune responses;
3. Restoring exhausted HBV-specific T cells with a novel non-blocking PD1 Ab;
4. Expanding recovered T cells with novel therapeutic vaccines.
The virology and immunology platforms will identify biomarkers and correlates of cure in experimental samples from the PoC clinical trial and from the humanized mouse model, that will be validated in real-life patient cohorts and especially in subgroup of patients that achieve a functional cure either spontaneously or with the currently available treatments.
During the program, some immune interventions will be ready to be investigated in the PoC clinical trial, while others will be evaluated in parallel in our preclinical models. The new knowledge generated in the proposed studies will inform strategic decisions in the selection of the best combination strategies to be tested in future clinical trials after the end of the EU funding.
To achieve this overall aim, an intensive work plan will be put in place. It foresees 8 interrelated work packages. For further information on the work plan developed in the IP-cure-B project, and the way in which the proposed plan will advance the field, do not hesitate to contact the project managers.